Insulin intensification from basal to basal-bolus resulted in significant glycemic improvement in both cohorts.
Insulin delivery with V-Go was associated with a greater reduction in A1C and required less insulin compared to intensification using multiple daily
injections (MDI)1
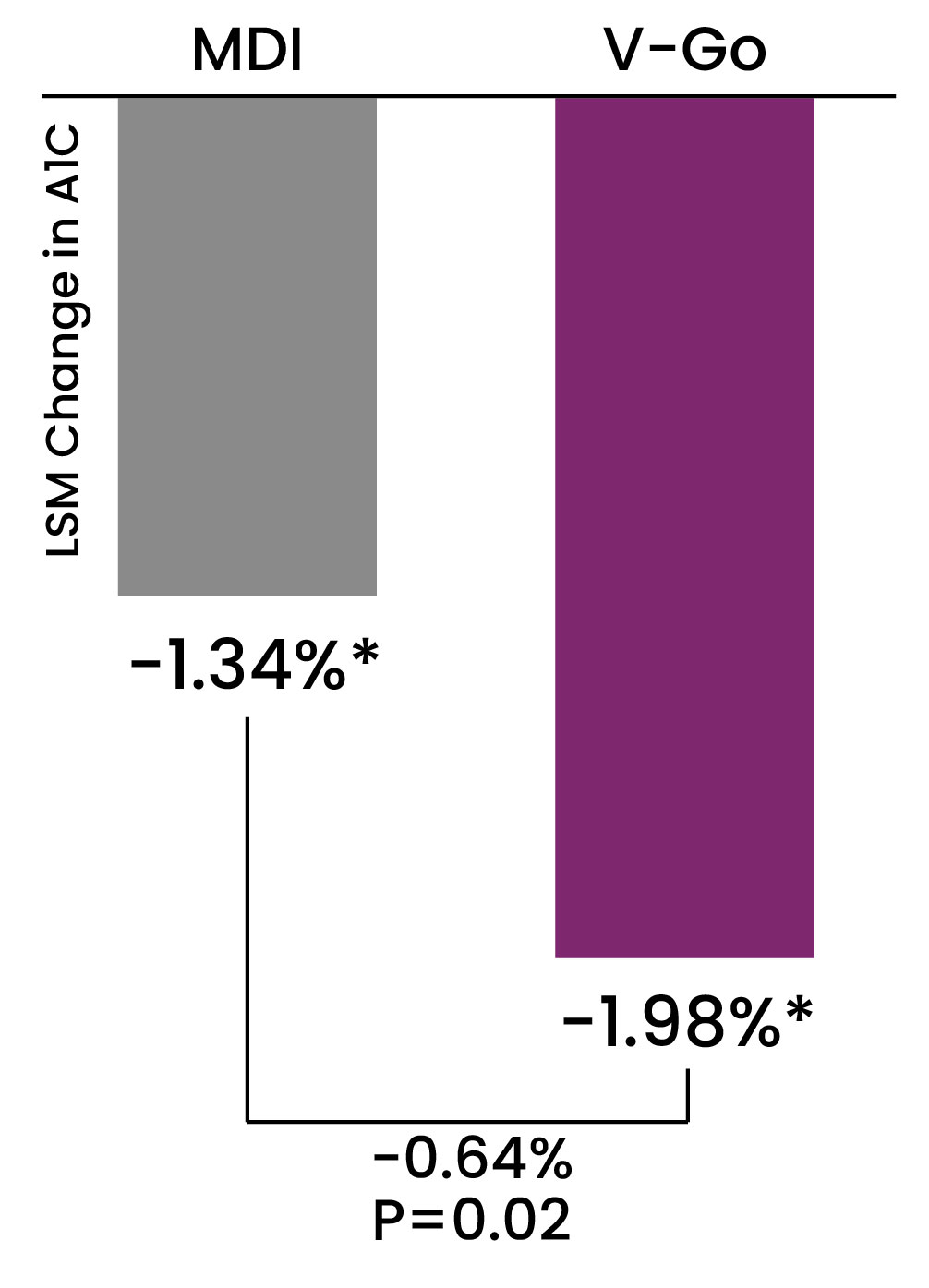
*P<0.001 compared to baseline after 27 weeks of therapy
Data from VALIDATE 2: A retrospective comparator evaluation in 116 patients to compare two methods of delivering intensified insulin therapy (IIT) in patients with type 2 diabetes inadequately controlled on basal insulin ± concomitant antihyperglycemic agents in a real-world clinical setting. Methods: Data for this retrospective study were obtained using electronic medical records from a large multicenter diabetes system. Records were queried to identify patients transitioned to V-Go or MDI using an insulin pen to add prandial insulin when A1C was >7% on basal insulin therapy. The primary endpoint was the difference in A1C change using follow-up A1C results. Baseline Mean A1C: V-Go Cohort 9.5%, MDI Cohort 9.4%. Baseline Basal Insulin Dose U/day: V-Go 51 U, MDI Cohort 46 U.1
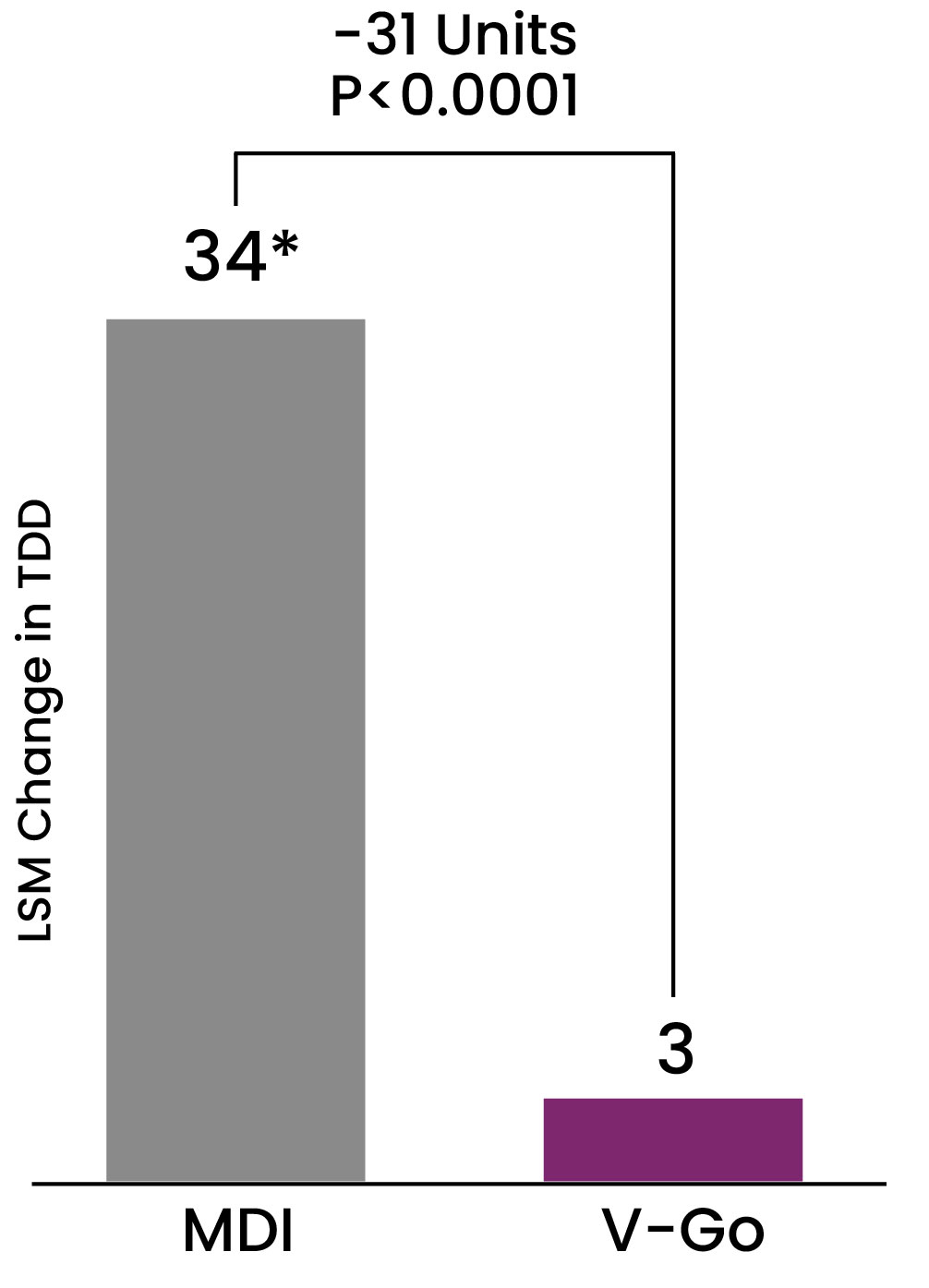
Multiple studies have proven switching from MDI to V-Go results in significant glycemic improvement and a significant reduction in prescribed insulin.
V-Go offers an efficient method of insulin delivery that improves clinical outcomes.2,3
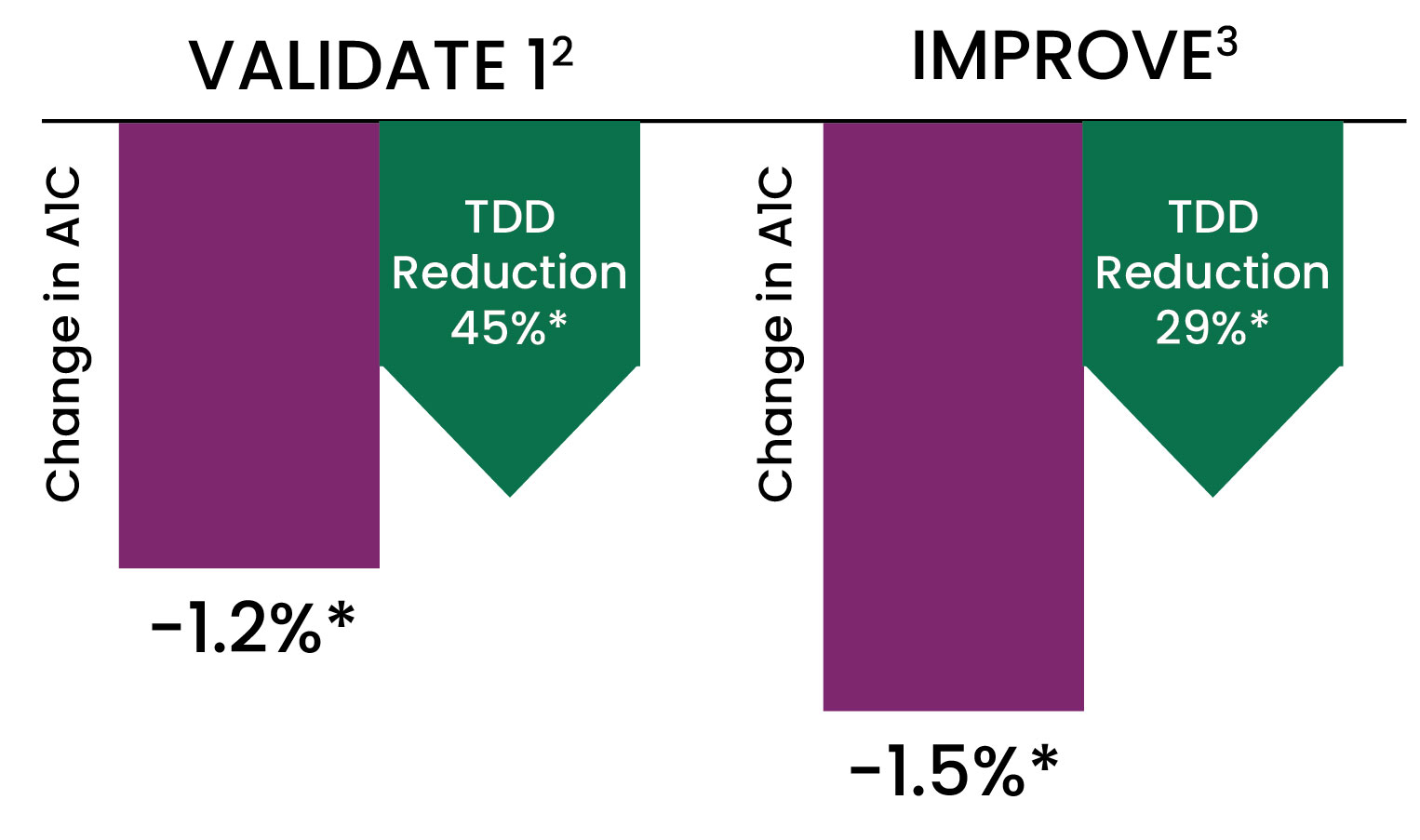
*P<0.001 compared to baseline
Data from VALIDATE 1: A retrospective analysis conducted in 153 patients across 13 diabetes specialty centers in Texas. Objective to evaluate the effect on A1C and TDD when switching from prior regimen to insulin delivery with V-Go in patients with diabetes.2
IMPROVE: A single-center, real world evaluation over time in 103 patients with diabetes. Objective to evaluate the impact of V-Go on A1C and TDD in patients with diabetes suboptimally controlled.3
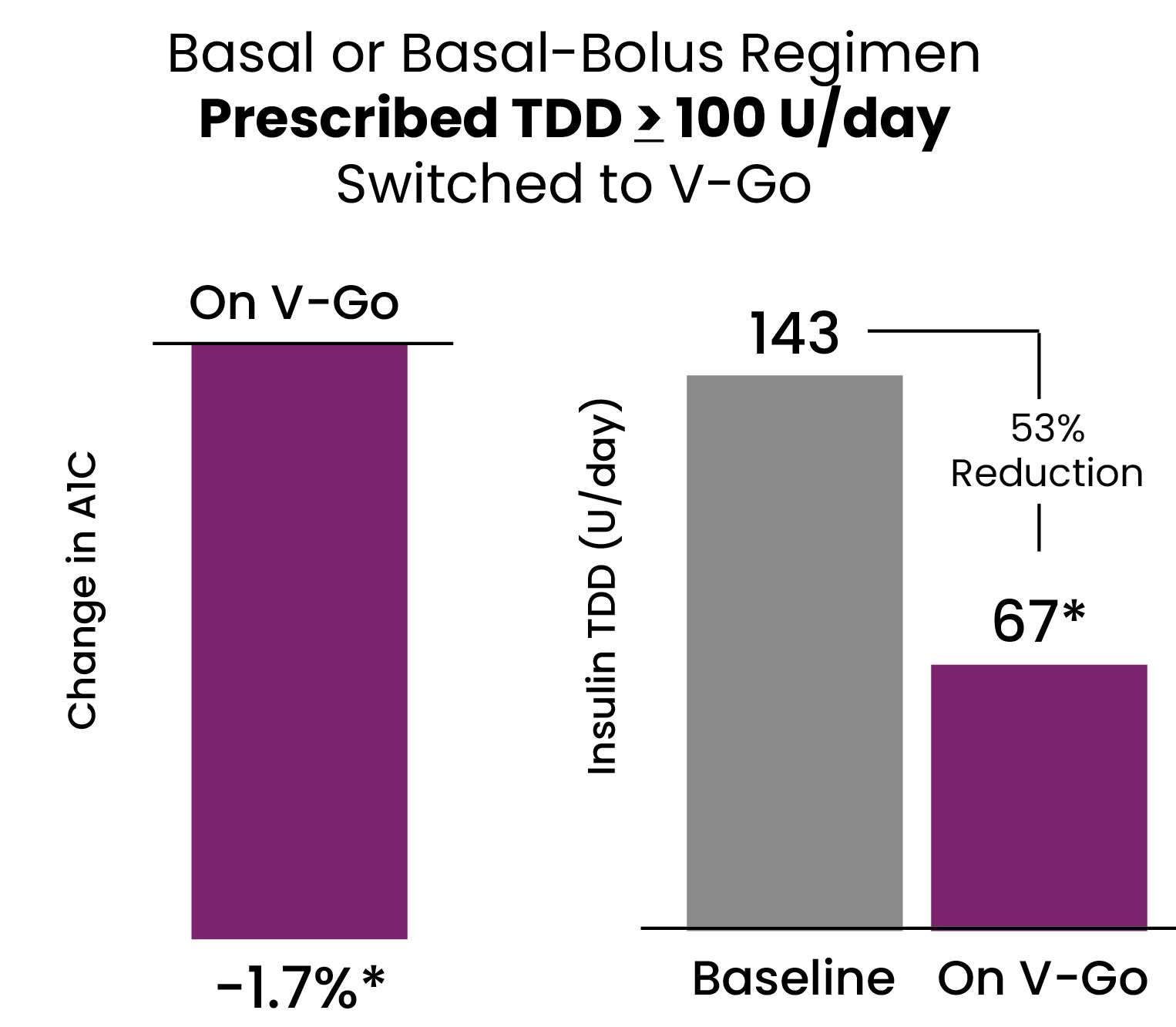
Data shown represents the cohorts of patients prescribed basal-bolus multiple daily injections (MDI) prior to switching to V-Go.
Results after 6 months and 14 months of V-Go use for VALIDATE 1 and IMPROVE, respectively.
Baseline mean A1C= VALIDATE 1: 9.4%, IMPROVE: 9.7%. Baseline mean insulin total daily dose (TDD) U/day= VALIDATE 1: 110 U (range 31 to 310 U), IMPROVE: 99 U (Range- 32 to 200 U).
This prospective, open-label, multicenter study recruited patients with type 2 diabetes (T2D) and suboptimal glycemic control (HbA1c ≥ 7%) across 28 centers. Efficacy analyses were conducted for all patients with a post-baseline HbA1c and results stratified based on prior antihyperglycemic medication therapies. Insulin dosing was at the discretion of the health care provider and the protocol did not mandate glycemic targets. Treatment satisfaction surveys were utilized to gain patient feedback on the use of V-Go.
Electronic health records were queried to identify patients meeting inclusion criteria. Study objectives evaluated change in A1C and insulin TDD compared to baseline. A total of 283 patients were enrolled across 9 diabetes specialty sites.
This study evaluated the change in A1C and insulin total daily dose (TDD) in a suboptimally-controlled (not achieving A1C targets) T2D population after switching to V-Go. A retrospective chart analysis at a diabetes clinic was performed to evaluate change in A1c measurements from baseline (V-Go initiation) to end of study observation.
A retrospective review of an outpatient clinic’s records was performed. Patients initiating V-Go with documented persistent use of V-Go or resumed persistent use of CID after short-term V-Go use were included (≥5 months of persistency). Baseline data and a total of two post-V-Go or CID initiation visits were examined for clinical and economic outcomes. Cost-effectiveness of each therapy was calculated by dividing the mean cost difference (baseline to office visit 2) by the mean change in A1c (baseline to office visit 2).
This prospective pragmatic clinical trial evaluated V-Go, a wearable insulin delivery device, compared with standard treatment optimization (STO) among insulin-treated patients with T2DM in a real world, community-based practice setting.
This descriptive study involved retrospective electronic medical record (EMR) review of all patients prescribed a V-Go device from April 2017 to May 2019. The target population was from two rural hospital-owned family medicine clinics in the southeastern United States. The clinics included four physicians and three nurse practitioners and are part of a network of 26 outpatient clinics. Adults ≥ 21 years of age with type 2 diabetes who had at least one A1C result 3 months after starting V-Go were included.
To examine glycemic control, insulin use, and diabetes medication costs for users of the V-Go Wearable Insulin Delivery device compared with MDI insulin therapy among individuals with T2DM in a commercially insured population in the United States.
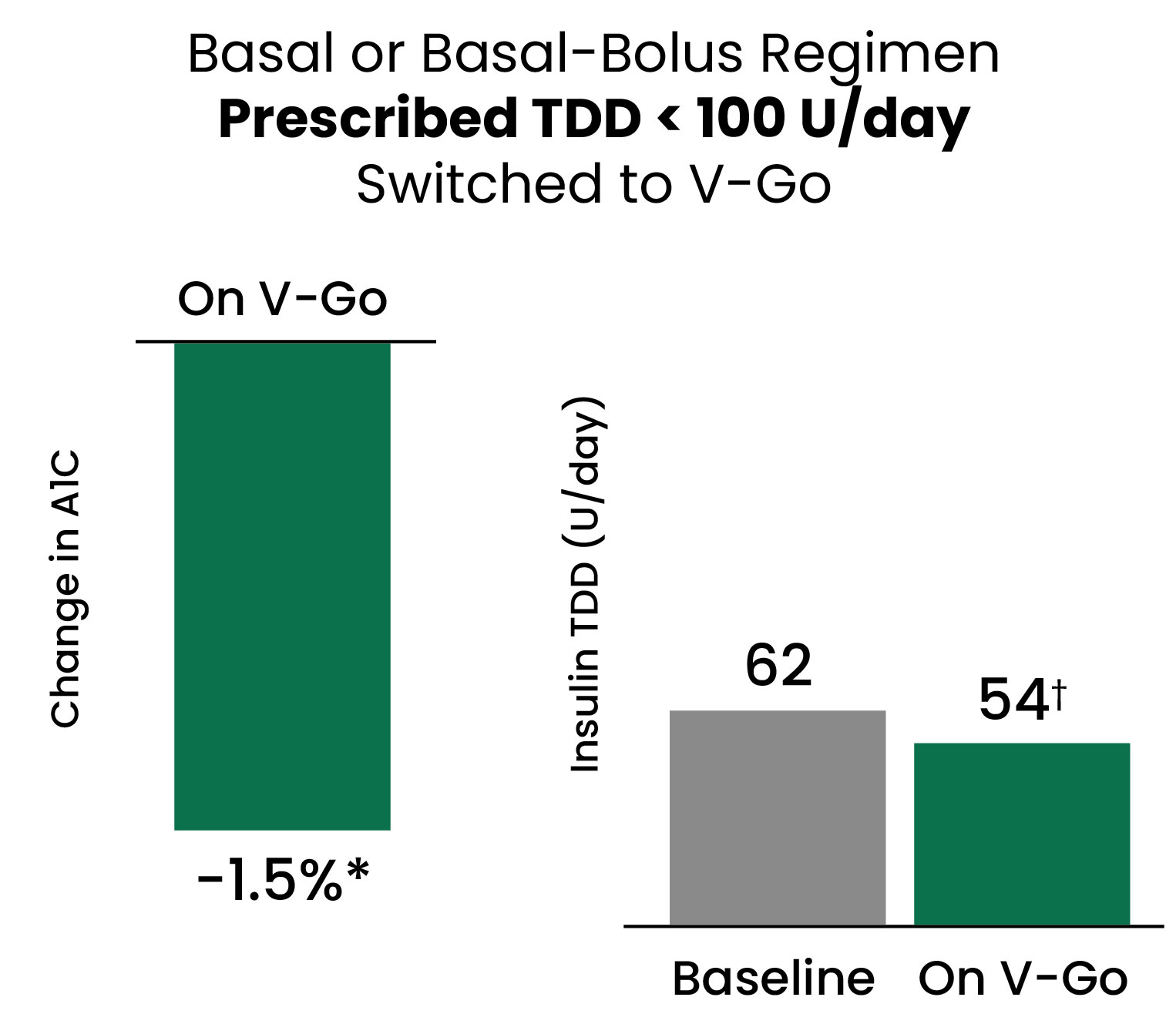
Electronic medical record data was obtained for adult patients with A1C > 7% treated at a multicenter endocrine practice who initiated V-Go between August 2012 and August 2015. Data were collected at baseline and for up to four follow-up visits, and were analyzed overall, stratified by insulin use at baseline, and for patients prescribed a basal-bolus insulin regimen delivered by multiple daily injections (MDI) at baseline. Economic evaluations were conducted in patients previously prescribed MDI regimens.
Fifteen (15), most of whom were non-insulin naïve, were initiated on V-Go and advised to record blood glucose four times daily (fasting and 2-hour postprandial breakfast, lunch and dinner) for 4 weeks. At weekly intervals, the physician titrated bolus doses of insulin, based on 2-hour postprandial averages, respectively. Basal doses were adjusted after bolus dosing was optimized. Clinical outcomes were evaluated after 4 months.
The main objective of this prospective active comparator study was to observe the A1C lowering effects of multiple daily insulin injections (MDII) versus the use of the V-Go insulin delivery system for patients with uncontrolled type 2 diabetes mellitus over a 3-month period. In addition, the effect on insulin requirement for these patients was assessed with secondary comparisons of weight, blood pressure, prevalence of hypoglycemic events, and quality of life before and after 3 months of intensified insulin therapy with regular monitoring by a clinical pharmacist at an internal medicine clinic.
To evaluate if switching patients prescribed subcutaneous insulin injections to V-Go for insulin delivery would impact clinical and economic parameters in patients with poorly controlled diabetes (A1C > 9 %).
Data for this retrospective study were obtained using electronic medical records from a large multicenter diabetes system. Records were queried to identify patients transitioned to V-Go(®) disposable insulin delivery device (V-Go) or multiple daily injections (MDI) using an insulin pen to add prandial insulin when A1C was >7% on basal insulin therapy. The primary endpoint was the difference in A1C change using follow-up A1C results.
A retrospective analysis of electronic medical records was conducted to assess patients with sub-optimal glycemic control defined as a glycated hemoglobin (HbA1c) >7%, switched to V-Go. Blood glucose control defined as change from baseline in HbA1c, prescribed insulin doses, body weight, concomitant anti-hyperglycemic agents, and reported hypoglycemia were collected prior to switching to V-Go and during V-Go use.
Patients used the V-Go and answered telephone surveys about their perception of device use. Corresponding clinical data were retrospectively collected before V-Go initiation, after 12 weeks of use, at the end of treatment, and 12 weeks after discontinuation. Analyses were performed with nonparametric statistical tests.
In six subjects receiving once-daily subcutaneous (SC) injections of insulin glargine (≥15 U/day) with or without concomitant oral antidiabetic drugs, glargine was discontinued following a 3-day baseline phase. The V-Go was then applied to the lower abdomen of the subjects once daily for 7 days (days 1–3 inpatient, days 4–7 outpatient). Each V-Go provided a continuous 24-hour preset basal infusion rate of insulin aspart (0.6 U/h) and up to three daily prandial doses at mealtimes. Capillary blood glucose concentrations were measured at 11 time points per day during the baseline and inpatient phases and at 4 time points per day during the outpatient phase. Additionally, glucose profiles were measured continuously on all days.
References: 1. Lajara R, Davidson JA, Nikkel CC, Morris TL. Endocr Pract Jun 2016. 2. Lajara R, Nikkel C. Poster Presented at ACCE. May 2015. 3. Sutton D, Higdon C, Nikkel C, Hilsinger K. Advances in Therapy. May 2018. 4. Lajara R, Nikkel C. Poster presented at 22nd Annual ISPOR Meeting; May 2017; Boston, MA. 5. Lajara R, Fetchick DA, Morris TL, Nikkel CN. Diabetes Ther. Oct 2015. 6. Everitt B, Harrison HC Jr, Nikkel C, Laswell E, Chen AMH. Sept 2019. 7. Grunberger G, Rosenfeld CR, Bode BW, Abbott SD, Nikkel C, Shi L, Strange P. Drugs Real World Outcomes. Mar 2020. 8. Hundal R, Kowalyk S, Wakim A, Nikkel C, Sink Ii JH, Doyle M. Sep 2020. 9. Zeidan T, Nikkel C, Dziengelewski B, Wu S, Chen AMH. Pharmacy. Nov 2020. 10. Rosenfeld CR, Bohannon NJ, Bode B, Kelman AS, Mintz SN, Schorr AB, Sandberg MI, Nambi S, Agarwala SK, Leichter SB, Larrabee B, Shi L, Strange P. Sept Endocr Pract. 2012.
US-VGO-0111